Immunohistochemistry (IHC) uses antibodies to detect and analyze protein expression while maintaining the composition, cellular characteristics, and structure of native tissues. IHC can be particularly helpful in diagnosing abnormalities in diseases such as cancer where tumor samples are available and contextual data is required.

Immunohistochemistry (IHC) slide preparation.
As implied by the names, the difference between immunocytochemistry (ICC) and immunohistochemistry (IHC) is whether you are detecting the antigen of interest in cells (ICC) or tissue sections (IHC). ICC can be very useful for determining whether cells express a certain protein while also providing subcellular localization and can be used in multiplex format to determine whether multiple proteins are co-located.
While IHC utilizes tissue sections that are either frozen or paraffin embedded, ICC is used to demonstrate the presence and location of proteins in cells. ICC can utilize cultured cell lines that are either adherent or, less commonly, in suspension or cells isolated from a human or animal source.
Similarly to IHC, ICC can be detected chromogenically or fluorescently. Chromogenic detection, an enzymatic method utilizing horseradish peroxidase (HRP) to convert a substrate such as DAB into a colored product that enables visualization of the antigen, has been used traditionally. However, fluorescent detection methods have gained widespread popularity in ICC applications due to ease of multiplex labeling and are rapidly gaining popularity in IHC.
On the CST website and elsewhere in scientific literature, this distinction can be confusing. Here at CST, you will find antibodies validated for ICC with fluorescent detection listed as IF/IC.
Western blotting (WB), also called immunoblotting, is a widely used and accepted technique to detect levels of protein expression in a cell or tissue extract. WB measures protein levels in a biological sample through antibody binding to a specific protein of interest. The precise binding that occurs between an antibody and its target protein epitope allows detection of highly specific amino acid sequences within a protein.
WB is extremely sensitive quantitatively and can detect very small quantities of protein. In contrast, the benefit of IHC is in the context of intact tissue. WB is often used as a complementary assay to confirm antibody specificity and provide more quantitative analysis of protein levels. However, the epitope recognized by the primary antibody may not be identically available in WB and IHC assays.
To browse WB-validated primary antibodies and companion products, click here.
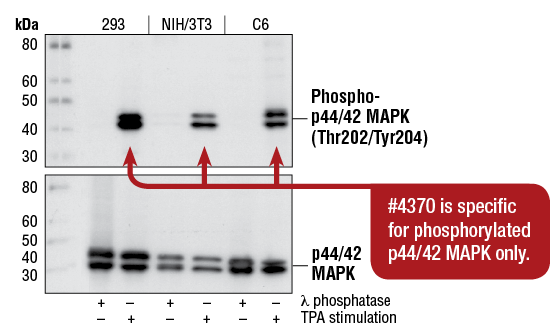
Phospho-p44/42 MAPK (Erk1/2) (Thr202/Tyr204) (D13.14.4E) XP® Rabbit mAb #4370: WB analysis of extracts from 293, NIH/3T3, and C6 cells, treated with λ phosphatase (to inhibit phosphorylation) or TPA #4174 (to stimulate phosphorylation), using #4370 (upper), or p44/42 MAPK (Erk1/2) (137F5) Rabbit mAb #4695 (lower).
As described above, IHC is used to detect and analyze protein expression in tissue samples with the benefit of maintaining the composition, cellular characteristics, and structure of the native tissue sample. In contrast, WB is used to detect levels of protein expression in a cell or tissue extract.
Detection of WB results can be chemiluminescent or fluorescent. Chemiluminescent detection offers ease of use while fluorescent detection offers digital data storage and ease of quantification. WB detection using fluorescent-labeled secondary antibodies requires specialized instrumentation, such as a fluorescent imager. Fluorescent detection is becoming increasingly popular as it provides faster visualization and quantification of results, as well as digital data storage. Fluorescent detection also allows phospho- and total protein levels to be determined on the same membrane, and regulation of different targets at different molecular weights can be examined simultaneously.
To browse WB detection reagents, click here.
CST has applied its antibody expertise to identify antibody pairs with optimal activity in solid phase sandwich enzyme-linked immunosorbant assays (ELISA). These assays enable the detection of low amounts of target protein from cell lysates.
In general, ELISA assays are more sensitive quantitatively than IHC assays. However, IHC assays provide results in context giving a semiquantitative overview of the tissue. ELISAs can also be set up to perform in a relatively high throughput manner and are very frequently available as a kit with the optimal protocol included. To browse ELISA kits, click here.
ELISA assays can be performed using a relatively broad range of sample types, including serum, plasma, cell culture media, etc. However, individual ELISA kits may only be validated for specific sample types, and this information will be outlined on the data sheet. As described above, IHC assays utilize tissue sections to demonstrate protein expression in context.
ELISA assay results can be detected using a direct or indirect approach.
In a direct ELISA assay, antibodies are labeled with alkaline phosphatase (AP) or HRP. HRP and AP substrates typically produce a colorimetric output that is read by a spectrophotometer. Detection can also occur by fluorescently labeled antibodies.
In an indirect ELISA, antibodies are coupled to biotin, followed by a streptavidin-conjugated enzyme step. It is also possible to use unlabeled primary antibodies followed by enzyme-coupled or biotinylated secondary antibodies. If the secondary antibody is biotinylated, then another step is required for detection. Here, treatment with the streptavidin-enzyme conjugate is followed by an appropriate substrate.