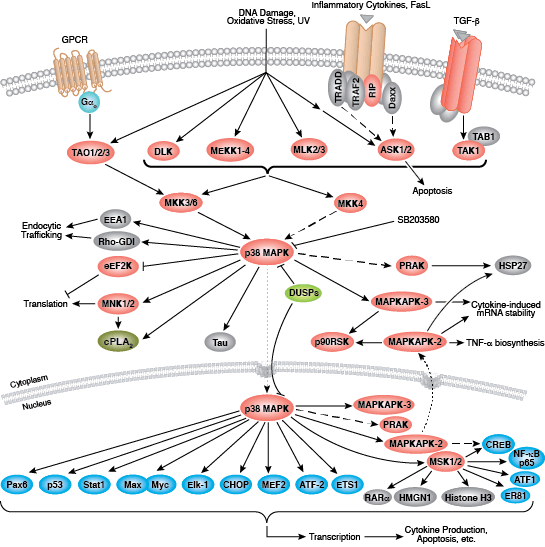
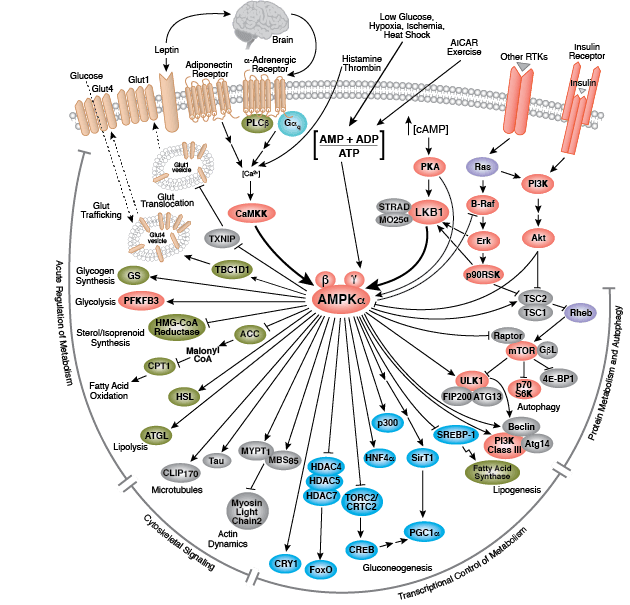
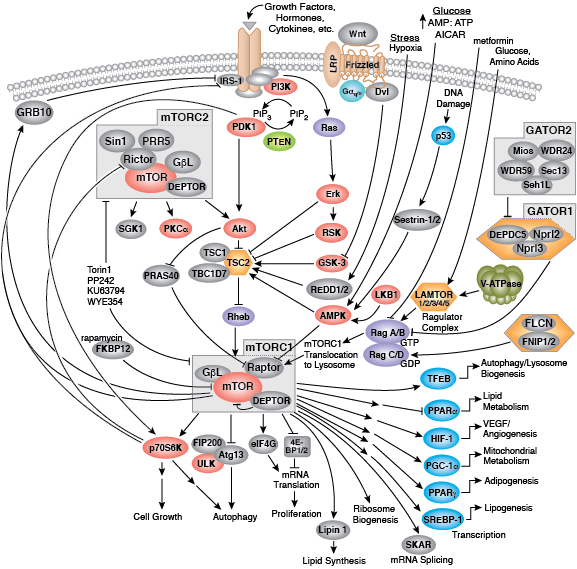
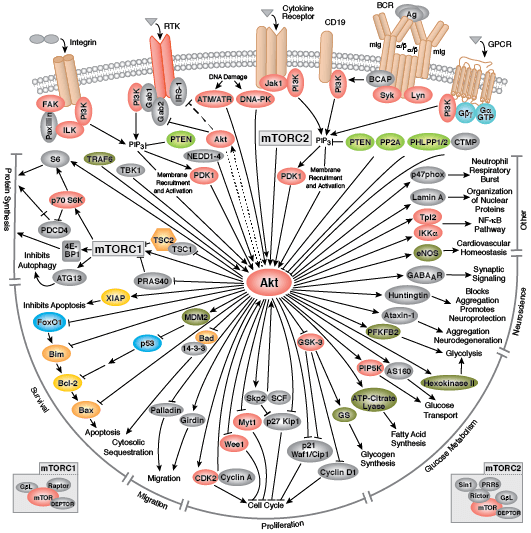
CST signaling pathway diagrams allow you to click on individual nodes to find research resources or product information. You can also download the pathway diagrams for educational and research purposes.
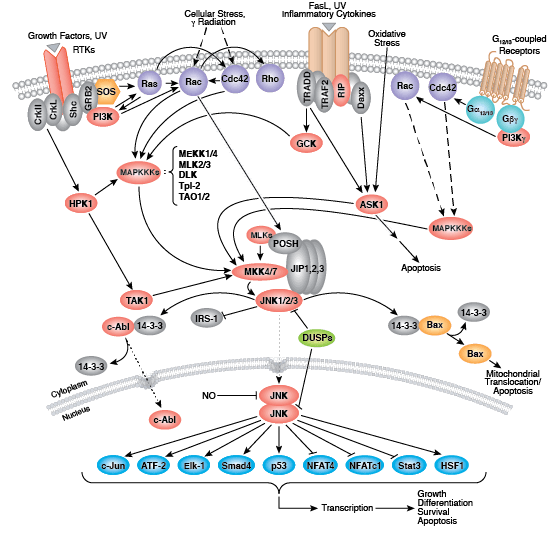
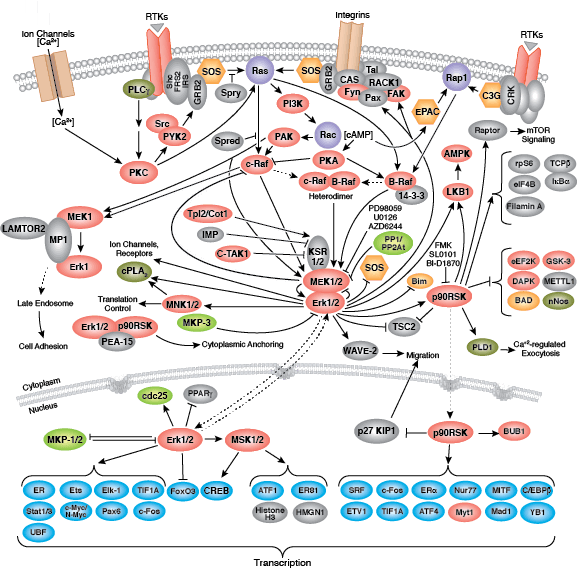
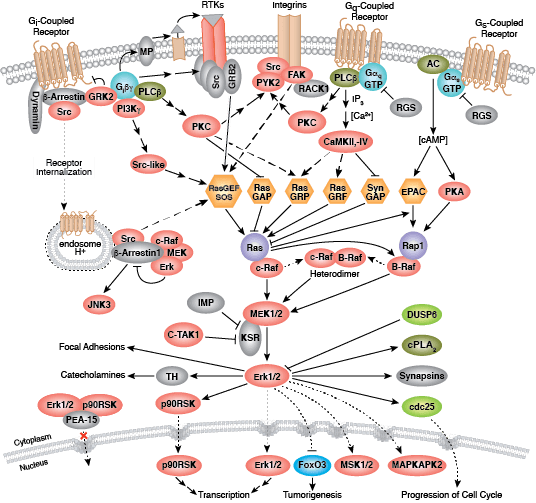
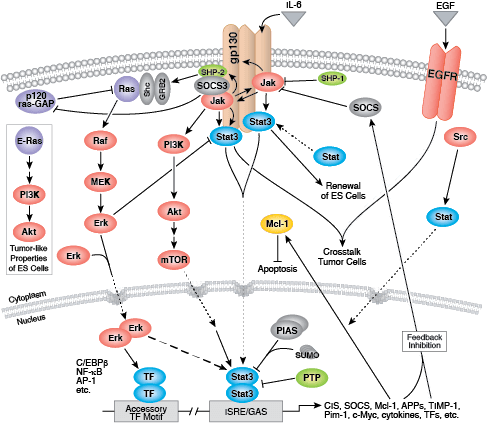
Whether functioning as cell surface receptors or as internal effectors of cell signaling, tyrosine kinases control multiple aspects of cell and organism growth, differentiation, and function. Approximately 20 receptor tyrosine kinase (RTK) families and at least 9 distinct groups of nonreceptor tyrosine kinases have been identified in humans. Regardless of localization, all tyrosine kinases regulate target protein function through transfer of phosphate from ATP to the hydroxyl group of a target protein tyrosine.
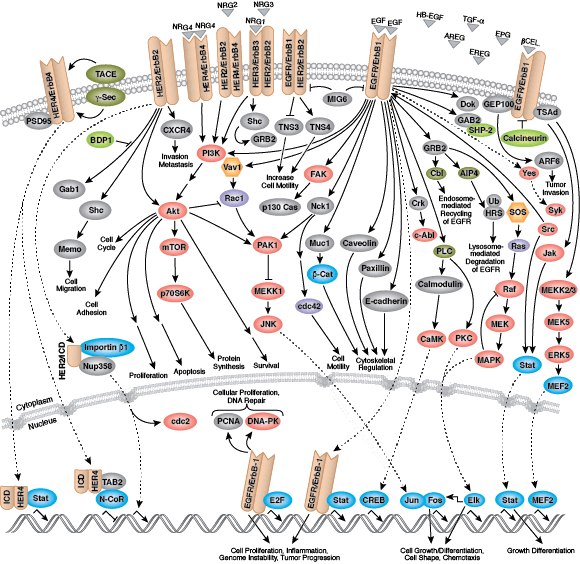
Most receptor tyrosine kinases are single-pass, transmembrane proteins that bind extracellular polypeptide ligands (i.e. growth factors) and cytoplasmic effector and adaptor proteins to regulate biological processes. Ligand binding promotes receptor dimerization and autophosphorylation of receptor tyrosine residues. The resultant conformational change stabilizes the active kinase, and subsequent phosphorylation events form binding sites for downstream adaptor, scaffold, and effector proteins.
Epidermal growth factor receptor (EGFR) is a well-studied tyrosine kinase and member of the ErbB receptor family. ErbB proteins bind extracellular ligands and form either homodimers or heterodimers with other family members. EGFR and ErbB4 bind a broad range of ligands, while ErbB2 is an orphan receptor with no identified ligand. ErbB3 lacks an active kinase domain and requires dimerization with a different ErbB family member for activation. Following ligand binding, phosphorylation of kinase domain activation loop residues maintains enzyme activity and provides a binding surface for substrate proteins.
Multiple adaptor and effector proteins bind sites within the carboxy-terminal tail of an active kinase. MAPK/Erk signaling follows binding of the GRB2 adaptor protein to activated EGFR at phospho-Tyr1068 and Shc scaffold protein to phosphorylated EGFR residues at Tyr1148 and Tyr1173. The adaptor protein c-Cbl binds EGFR at Tyr1045 to promote EGFR receptor ubiquitination and degradation.
Non-receptor tyrosine kinases include many well-characterized proteins (such as the Src family kinases, c-Abl, and Jak kinases) as well as other kinases that control eukaryotic cell growth and differentiation. Tec family kinases (Tec, Btk, Etk, Itk, and Txk) are essential in B cell and T cell receptor signaling and contain a unique amino terminal pleckstrin homology domain that helps recruit the kinase to the plasma membrane. Src family kinases play essential roles in well-defined activating and inhibitory pathways in the innate immune response. Unrelated kinases FAK, Fer, and ACK1 each regulate cell movement and migration, while TNK1 acts in pathways that negatively regulate cell growth.
Zap-70 and Syk are involved with different aspects of the immune response, while Fes plays a role in hematopoiesis.